Abstract
Introduction: Extramedullary multiple myeloma (EMM) is an aggressive manifestation of multiple myeloma (MM) reported to occur in approximately 7% of patients at diagnosis and up to 30% of patients at relapse. EMM is characterised by the spread of malignant plasma cells from the bone marrow microenvironment to distal tissues or organs. It is associated with an adverse prognosis, correlating with a significant reduction in overall survival. Currently there are no validated, established biomarkers to predict EMM. Furthermore, EMM is often treated similarly to high-risk MM with no targeted therapeutic strategies. In-depth proteomic studies on EMM are lacking and the underlying molecular mechanisms that facilitate extramedullary transition are yet to be fully defined. Novel biomarkers and therapeutic targets are urgently required. To enhance our understanding of EMM and to identify novel prognostic biomarkers, we performed a mass spectrometry-based proteomic study on plasma from MM patients with and without extramedullary spread.
Methods: Label-free liquid chromatography mass spectrometric analysis of age and gender-matched medullary MM (n=8) and EMM (n=9) blood plasma samples was carried out using a Thermo Q-Exactive mass spectrometer (Thermo Fisher Scientific). Proteome Discoverer 2.2 using Sequest HT (Thermo Fisher Scientific) and a percolator were employed for the identification of peptides and proteins. Several parameters were defined for protein identification: MS/MS mass tolerance was set to 0.02 Da; peptide mass tolerance was set to 10ppm; methionine oxidation was set as a variable modification; carbamido-methylation was set as a fixed modification; and up to two missed cleavages were tolerated. Peptide probability was set to high confidence. Data was imported into Perseus (1.6.14.0) for further analysis. Proteins with less than 70% valid values were removed from the analysis. Proteins of interest were identified based on an FDR-adjusted p-value ≤0.1, fold change >1.5 between experimental groups. Six proteins were selected for further validation using DuoSet enzyme linked immunosorbent assay (ELISA) kits (R&D Systems). We performed receiver operating characteristic (ROC) and area under the curve (AUC) analyses to determine the diagnostic potential of the validated proteins.
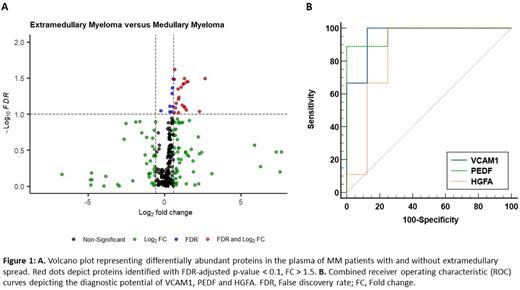
Results: The median age was 65. Survival analysis revealed a statistically significant change in overall survival (OS) between the two patient cohorts (Log-rank = 3.977, P = 0.046). The median OS of patients with EMM and those without extramedullary spread was 19 months and 83 months, respectively. Our quantitative MS-based proteomic analysis identified 21 proteins of differential abundance between EMM and MM patient plasma (False discovery rate (FDR)-adjusted p-value < 0.1, fold change > 1.5) (Fig. 1A). Antibody-based validation using ELISAs was performed on six proteins (vascular cell adhesion molecule 1 (VCAM1), hepatocyte growth factor activator (HGFA), pigment epithelial-derived factor (PEDF), alpha-2-macroglobulin (A2M), cholinesterase (BCHE), aminopeptidase N (CD13)). VCAM1, HGFA and PEDF were confirmed as being significantly altered between the two cohorts (FDR-adjusted p-value < 0.05). VCAM1, HGFA and PEDF were subject to ROC analyses, demonstrating high discriminatory power for EMM diagnosis (AUC = 0.96, AUC = 0.85, and AUC = 0.97, respectively). The diagnostic efficacy was further enhanced by combining these biomarkers using a logistic regression model (AUC = 1).
Conclusion: Our mass spectrometry and antibody-based study identified proteins of differential abundance in the blood plasma of MM patients with and without extramedullary spread. VCAM1, PEDF and HGFA represent promising predictive biomarkers and warrant further investigation in a larger cohort of patients.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.